Abstract
Introduction Primary (diffuse) large B-cell lymphoma of the testis (PTL) is an aggressive extranodal B-cell lymphoma characterized by frequent relapses in the central nervous system (CNS) and other extranodal sites. While PTL has now been recognized as a distinct lymphoma in the latest classifications (International Consensus Classification and WHO HAEM5), however, the allowable extent of involvement outside of the testes is unclear from these publications. PTL are largely assigned to the MCD genetic subtype of diffuse large B-cell lymphoma (DLBCL) by LymphGen with frequent MYD88L265P mutations and have 6p loss associated with immune evasion. BCL6 rearrangements (BCL6-R), a feature of BN2, have been associated with an increased risk of CNS relapse (Twa et al., Blood 2021), though the mechanisms enabling tumor establishment in the CNS remain unclear. Here, we provide a characterization of the genomic landscape of DLBCL involving the testes, aiming to understand whether systemic disease is distinct from disease isolated to the testes and whether there are genetic features associated with patient outcomes.
Methods Patients with DLBCL involving the testis, treated with R-CHOP immunochemotherapy, were identified in the BC Cancer Lymphoid Cancer database, yielding archival formalin-fixed paraffin-embedded orchidectomy samples from 65 patients. BCL6-R were identified by break-apart fluorescent in situ hybridization (FISH). High confidence simple somatic mutations (SSMs) from whole exome sequencing were selected using a voting approach of four variant callers (Strelka2, Lofreq, Mutect2, SAGE). Candidate copy number alterations (CNAs) were identified using CNVkit and PureCN and high confidence CNAs were determined using GISTIC2.0. LymphGen was run with SSMs to annotate genetic subgroups. Clinical endpoints included time to progression (TTP), disease-specific survival (DSS) and CNS relapse cumulative risk.
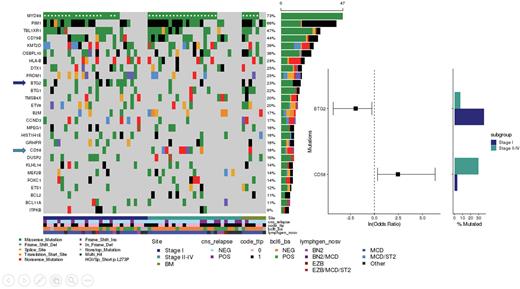
Results The study cohort had a median age of 72 years (range 26-93) and median follow-up of living patients was 5.5 years. In 30 patients lymphoma was isolated to the testes (Stage IE), 27 had concurrent involvement outside of the testes (Stage II/E-IV), and eight had DLBCL isolated to the testes with low grade lymphoma in the bone marrow (herein labelled "BM" - excluded from isolated vs systemic comparisons). BCL6-R were detected in 29% of the tumors. The majority of the tumors (69%) were assigned to the MCD subgroup by LymphGen with 73% and 66% of tumors harboring MYD88L265P and PIM1 mutations, respectively, though 40% of BCL6-R tumors were assigned to BN2 or Other.
To address whether there are biological differences between disease isolated to the testes versus those that also have systemic involvement, we compared tumors from patients with stage I vs II-IV disease. There were no significant differences in the proportions of BCL6-R or LymphGen subgroups. Similarly, there was no difference in CNAs and the mutational landscapes were largely the same, with the exception of two significant differences. There was a significant enrichment for CD58 truncating mutations in stage II-IV (31%; q < 0.1), with a missense mutation observed in one stage IE tumor. Secondly, BTG2 mutations were more frequently observed in stage I disease (37% vs 8%; q < 0.1).
We observed significantly superior TTP (Hazard ratio (HR) = 0.46, P < 0.05) and DSS (HR = 0.34, P < 0.01) in patients with stage I vs stage II-IV diseases. In stage II-IV disease, the MCD (n=18) (vs non-MCD (n=9)) subgroup was associated with significantly superior TTP (2-year 72% vs 22% TTP; HR = 0.36, P < 0.05) and DSS (2-year 72% vs 22% DSS; HR = 0.37, P < 0.05) and lower frequency of CNS relapse (13% vs 55% cumulative relapse at 2 years; HR = 0.23, P < 0.05). In contrast, LymphGen subgroup was not prognostic in stage I disease.
Conclusion Here, we show that there are very few genetic differences comparing testicular DLBCL isolated to the testes to those with stage II-IV disease. The novel observation of truncating mutations in CD58 exclusively in patients with systemic involvement suggests that these mutations contribute to immune evasion thereby promoting disease outside of the immune privileged site of the testes. Genetic subgroups appear to be associated with outcomes in patients with systemic involvement - an observation that requires validation in a larger cohort.
Disclosures
Slack:Seagen: Honoraria. Craig:Bayer: Consultancy; BeiGene: Honoraria. Sehn:Teva, Roche/Genentech: Consultancy, Honoraria, Research Funding; Chugai: Consultancy, Honoraria; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Debiopharm, Genmab, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Novartis, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Consultancy; AbbVie, Acerta, Amgen, Apobiologix, AstraZeneca, BMS/Celgene, Gilead, Incyte, Janssen, Kite, Karyopharm, Lundbeck, Merck, Morphosys, Sandoz, Seattle Genetics, Servier, Takeda, TG Therapeutics, Verastem: Honoraria. Villa:AstraZeneca, Roche: Research Funding; Roche, AstraZeneca, Abbvie, Janssen, Kite/Gilead, BMS/Celgene, BeiGene, Kyowa Kirin: Consultancy, Honoraria. Gerrie:Sandoz: Honoraria; AbbVie: Honoraria, Research Funding; AstraZeneca: Honoraria, Research Funding; Janssen: Honoraria, Research Funding. Steidl:Abbvie: Consultancy; Bayer: Consultancy; Bristol Myers Squibb: Consultancy; Curis Inc: Consultancy; Epizyme: Research Funding; Roche: Consultancy; Seattle Genetics: Consultancy; Trillium Therapeutics: Research Funding. Scott:Incyte: Consultancy; Abbvie: Consultancy; AstraZeneca: Consultancy, Honoraria; Janssen: Consultancy, Research Funding; Roche: Research Funding; NanoString: Patents & Royalties.
Author notes
Asterisk with author names denotes non-ASH members.

This feature is available to Subscribers Only
Sign In or Create an Account Close Modal